-

About the MSI-plus assay
The test screens for Lynch syndrome, a hereditary condition which brings increased risk of certain types of cancer, including colorectal. -

How it works
The assay detects a pattern of chemical changes on DNA which can indicate the condition. -

What’s in the kit
It is the first product of Newcastle Hospitals to be UKCA-marked, meaning it is certified to be sold in the UK. -
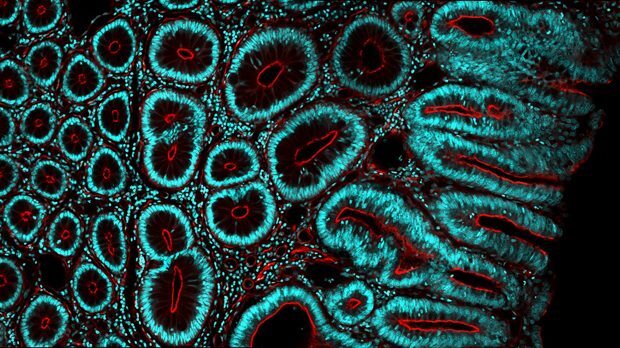
Real world impact
The assay has facilitated testing of over 95% of the 2,500 colorectal cancers in three million people in the north east and north Cumbria. -

Regulatory approvals
Registered with the MHRA and validated with UKAS accreditation. -

Contact us
We are currently engaging with collaborators and potential customers to expand the adoption and impact of the MSI-plus assay.
