The project
Cutaneous melanoma is a form of skin cancer with an increasing worldwide incidence with over 17,000 patients diagnosed each year in the UK. This type of cancer is more likely than other cancers to spread to other parts of the body if it isn’t diagnosed and treated early.
Prior to new techniques to identify low risk melanoma patients, all early stage one and two patients required monitoring with multiple visits to outpatient clinics over five years.
Biomarkers can identify those patients who are truly at low-risk of their disease spreading, allowing a more conservative follow-up regime. While those identified at risk of metastasis can receive timely treatment.
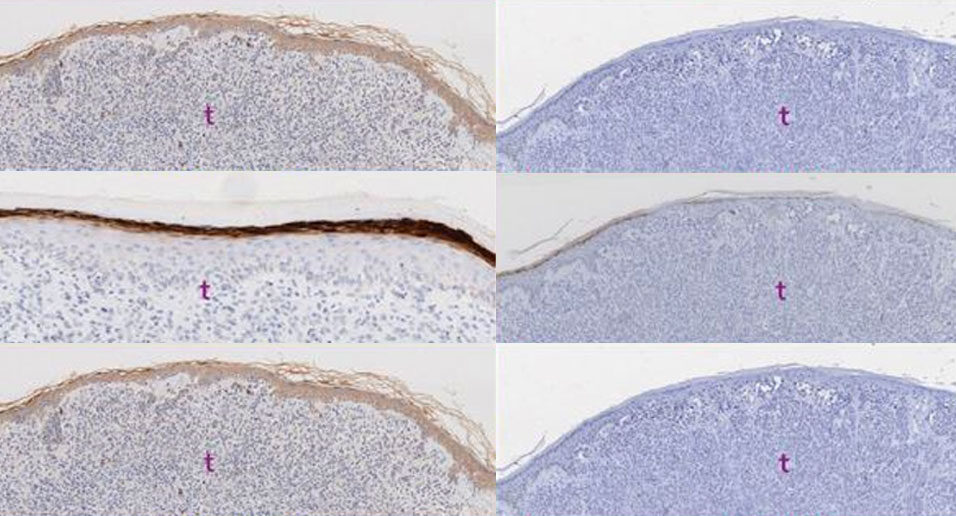
Biomarker discovery and validation studies at Newcastle University led to the identification of two proteins, AMBRA1 and loricrin, which give an indicator of these low-risk patients. AmLo Biosciences produced an assay kit containing AMBLor – the name for the combined AMBRA1 and loricrin antibodies.
How we helped
NovoPath is an established part of the cellular pathology department in the Royal Victoria Infirmary in Newcastle and we support research in ‘proximity’ to the clinical setting and towards development of stratified, translational medicine.
AMLo Biosciences is translating ground-breaking research in cancer biomarkers into a revolutionary range of diagnostic products to improve patient outcomes and reduce the financial burden of cancers on global healthcare economies.
We supported the set up and transfer of geographically distinct samples of the stage one and two melanomas from UK, Spain, Australia, and the USA and AMBLor analysis was completed on the UK and Spanish samples.
Two of our pathologists also reviewed and blind scored large internationally sourced melanoma samples from ethical biobanks using digital imaging to link with colleagues in Cambridge and Durham.
We have also supported a UKAS approved referral test for AMBRA1 and loricrin in advance of UK-CA marking of AMBLor, facilitating the commercial launch of a product earlier than planned. The UKAS referral service offered by Newcastle Hospitals is now available to patients internationally.
Outcome
We have completed the validation of the AMBLor diagnostic antibodies for stage one and two melanomas.
Our work supported the publication of a positive NICE MedTech information briefing for AMBLor as a prognostic test for low-risk, early-stage, non-ulcerated cutaneous melanoma.
The picture shows example images of IHC staining demonstrating maintenance and loss of AMBRA1 and Loricrin biomarkers.
Find out more by clicking this page.